When reviewing ex parte PTAB decisions, it very helpful to review both reversals and affirmances to see how issues morph in a case. A representative’s job is to make sure the issues morph in a way beneficial to their client. Likewise, the representative has to make sure that careless admissions are minimized as you can sometimes never predict how those can contribute to an easy affirmance as the case progresses.
A recent PTAB case handled by GlaxoSmithKline (GSK) is a good example of how things can go off track, even when the issues seem straightforward. The case relates to a phamaceutical composition, with claim 1 reproduced below:

The claim was rejected based on a combination of references, where the references did show all of the claimed components (among many others in big lists). GSK’s main argument was to argue that the examiner was picking and choosing using hindsight.
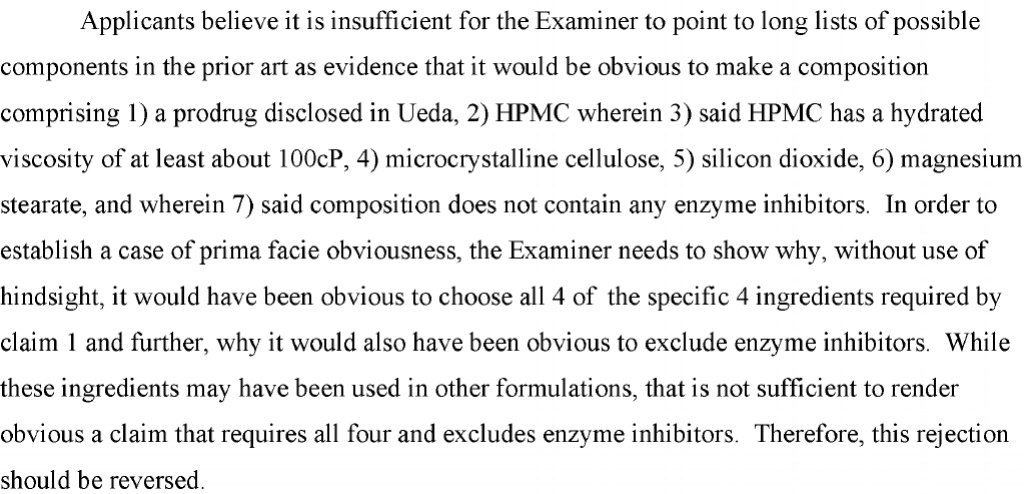
Now, GSK is probably right. The examiner used the claims as a guide to pick and choose the elements from the prior art. The problem is that, without more, this is a tough argument for the applicant to win on appeal. The Examiner did have some reasons for combining the references, and once you get past that, there does not seem to be much in the brief explaining why the particular combination of elements is inventive. The PTAB here did what could have been expected and affirmed the Examiner’s approach.
The one point GSK had was (7) above – a negative limitation that does not appear to be shown by the cited references. Normally, this is a great argument for an applicant on appeal where the examiner effectively ignores the feature. The problem for GSK here was that it effectively admitted that this element was shown in the art. Specifically, the brief for GSK includes the following statements:

GSK is correct for the positively claimed elements, so what harm is there in this admission? The harm is that the negative limitation does not appear to be clearly shown or even alleged to be explicitly shown in the references. Therefore, this admission kills what might otherwise be a significant point for the applicant. The PTAB attempts to push this issue under the rug. Specifically, the PTAB states that “the Examiner’s burden to show a prima facie case of unpatentability does not require the Examiner to prove that a person of ordinary skill in the art would not have included enzyme inhibitors; it is Appellants’ burden to show the opposite. Cf Upsher-Smith Labs., Inc. v. Pamlab, LLC, 412 F.3d 1319, 1322 (Fed. Cir. 2005) (quote omitted).” However, the PTAB leaves out some very important facts in Upsher. The full quote from the Federal Circuit’s opinion makes clear that the element in question was listed specifically as optional in the cited reference:
First, Upsher-Smith argues that the district court erred by placing the burden on Upsher-Smith to prove that the negative limitation was not found in the prior art rather than on Pamlab to prove the presence of the negative limitation in the prior art. The district court did not so err. A century-old axiom of patent law holds that a product “which would literally infringe if later in time anticipates if earlier.” Schering Corp. v. Geneva Pharms., Inc., 339 F.3d 1373, 1379 (Fed.Cir.2003) (quoting Bristol-Myers Squibb Co. v. Ben Venue Labs., Inc., 246 F.3d 1368, 1378 (Fed.Cir.2001)); accord Peters v. Active Mfg. Co., 129 U.S. 530, 537, 9 S.Ct. 389, 32 L.Ed. 738 (1889) (“That which infringes, if later, would anticipate, if earlier.”). The European Application’s “optional inclusion” of antioxidants teaches vitamin supplement compositions that both do and do not contain antioxidants. Thus, because compositions made according to the European Application that do not contain antioxidants would infringe the asserted claims of the ‘624 and ‘646 patents, those compositions anticipate the asserted claims despite no express teaching to exclude the antioxidants in the European Application. Consequently, Pamlab presented a prima facie case of anticipation, and the district court properly placed the burden on Upsher-Smith to present rebuttal evidence sufficient to raise a genuine issue of material fact of no anticipation by the European Application.
None of the briefs address the issue of whether a reference listed exzyme inhibitors explicitly as optional elements. So unfortunately for GSK, by effectively admitting that the only issue was one of motivation to combine, the PTAB could easily affirm the rejection and side-step the negative claim limitation by citing case law that may or may not even have been relevant.

Leave a comment